|
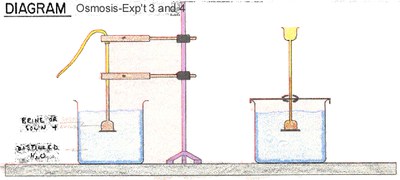
The osmosis demo uses the single-ended thistle tube only, as it is tricky to seal the dialysis membrane onto a wide opening.
- Soak dialysis membrane tubes in a beaker of distilled water.
- Using another small beaker, add food colouring to ~200ml distilled water
- Dissolve table salt into the coloured solution. Older demos suggest about 80g or 70g of salt for 200ml. (note: 1tsp ~6g of salt)
- Heat up the solution so the salt dissolves easier. Let cool.
- When the membrane is soft, tie off one end with a knot, elastic and / or fishing line.
- Open up the other end of the membrane tube and tie it around the narrow end of the thistle tube.
- Clip the thistle tube support stand onto the tube
- Prepare an empty beaker and set the thistle tube in it.
- When the coloured brine is at room temperature, inject the brine into the thistle tube / membrane pouch using the small syringe needle - this minimizes bubbles in the pouch.
- Squeeze the pouch to get rid of the air: beware of exploding brine as the bubbles escape!
- Fill a large beaker (1L or 2L) with distilled water.
- Rest the thistle tube on the large beaker so that the membrane is immersed completely in water.
- Tape a marker on the brine level along the thistle tube
- Leave the setup for a while, and observe changes in the brine level.
A glass funnel with a long neck may be used, with another membrane pouch full of distilled water, to contrast osmotic pressure behaviour with the brine.
Old Description:
A. To make brine solution of salt water
i. Mix 870 gm of salt water with 200ml of distilled water (final solution 64.5 gram salt, 190 ml water)
ii. Gently heat mixture to dissolve salt
iii. Filter mixture twice to remove undissolved particles
1. Use plastic syringe and Teflon tubing to fill membrane or plug narrow of thistle tube and then fill tube with brine solution and some Lugol solution
2. Cover the large opening completely with the clear membrane and seal it using elastic bands and fish line thread. Check for leaks and rinse with distilled water
3. Fill 900ml beaker with distilled water
4. Clamp membrane under water. The brime level in the tube should be at the bottom of the narrow opening
B. To make sugar solution
i. Mix 25ml of Lab-aids glucose solution and 25ml of Lab-aids liquid starch together
1. Plug one end of double thistle tube
2. Fill tube with sugar solution
3. Cover one opening completely with rubber membrane and seal it using elastic bands and fish line thread, Check for leaks and rinse with distilled water
4. Fill battery jar beaker with distilled water
5. Place tube between clamps and lower membrane into the water. The sugar level should be near the bottom of the narrow opening
Method:
1. Observe the level of the brine solution rise up 8cm in 1 hr and about 52cm in 12 hours
2. Observe the level of the sugar solution rise up 3cm in 1 hr and about 30cm in 12 hours
** note from Lilian: I'm not sure what the Lugol solution does with the brine: other documentation seem to suggest Lugol solution should be used with the sugar/starch solution instead, as it is a starch indicator (turns purple). Larry says put some ink in the brine (for visibility). Red food coloring worked well for me this last time. I tied off the tubular membrane into a sac for the brine solution, and cut out a flat piece to tie onto one end of the double thistle tube for the sugar. (Feb 2012)
Historical Diagram and Photo:

|